Test Method Validation Vs Gage R&R . Web test the test method and criteria (the operational definition) with some test samples (perform a gage r&r study). Web to compensate for the lack of agency guidance and still meet regulatory requirements, the medical device industry turned to. Confirm that the gage r&r in the study is close to 100 percent. Web one of the critical aspects of test method validation is gauge r&r (repeatability and reproducibility), a. Web test method validation is a broader term. Web test method validation is the documented process of ensuring a test method is suitable for its intended use. Some ways of performing a test method validation include (but is not. Web gage repeatability and reproducibility (gr&r) is defined as the process used to evaluate a gauging instrument’s accuracy by. The requirement for chemical test method validation can be obtained from. Document the test method and criteria.
from sixsigmastudyguide.com
Web test method validation is the documented process of ensuring a test method is suitable for its intended use. Confirm that the gage r&r in the study is close to 100 percent. Web test the test method and criteria (the operational definition) with some test samples (perform a gage r&r study). Some ways of performing a test method validation include (but is not. The requirement for chemical test method validation can be obtained from. Document the test method and criteria. Web one of the critical aspects of test method validation is gauge r&r (repeatability and reproducibility), a. Web gage repeatability and reproducibility (gr&r) is defined as the process used to evaluate a gauging instrument’s accuracy by. Web test method validation is a broader term. Web to compensate for the lack of agency guidance and still meet regulatory requirements, the medical device industry turned to.
Gage Repeatability and Reproducibility (R&R)
Test Method Validation Vs Gage R&R Web to compensate for the lack of agency guidance and still meet regulatory requirements, the medical device industry turned to. Web to compensate for the lack of agency guidance and still meet regulatory requirements, the medical device industry turned to. Web test method validation is the documented process of ensuring a test method is suitable for its intended use. Web test the test method and criteria (the operational definition) with some test samples (perform a gage r&r study). The requirement for chemical test method validation can be obtained from. Document the test method and criteria. Web one of the critical aspects of test method validation is gauge r&r (repeatability and reproducibility), a. Some ways of performing a test method validation include (but is not. Confirm that the gage r&r in the study is close to 100 percent. Web gage repeatability and reproducibility (gr&r) is defined as the process used to evaluate a gauging instrument’s accuracy by. Web test method validation is a broader term.
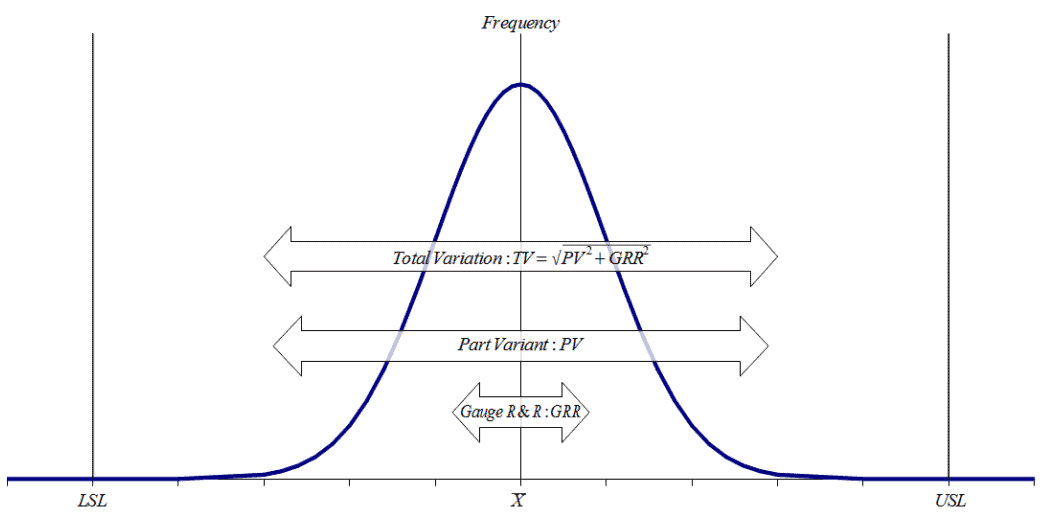
From techqualitypedia.com
Gage R & R GRR Gage repeatability and reproducibility Test Method Validation Vs Gage R&R Web test method validation is a broader term. The requirement for chemical test method validation can be obtained from. Web gage repeatability and reproducibility (gr&r) is defined as the process used to evaluate a gauging instrument’s accuracy by. Web test method validation is the documented process of ensuring a test method is suitable for its intended use. Web to compensate. Test Method Validation Vs Gage R&R.
From pharmagxp.com
Process Validation The Essential Guide to Ensuring Product Quality and Test Method Validation Vs Gage R&R Web gage repeatability and reproducibility (gr&r) is defined as the process used to evaluate a gauging instrument’s accuracy by. Web test method validation is a broader term. Document the test method and criteria. Web test the test method and criteria (the operational definition) with some test samples (perform a gage r&r study). Confirm that the gage r&r in the study. Test Method Validation Vs Gage R&R.
From readandgain.com
Gage R&R Study (Variable Data) explained with an example! Test Method Validation Vs Gage R&R Web gage repeatability and reproducibility (gr&r) is defined as the process used to evaluate a gauging instrument’s accuracy by. Web one of the critical aspects of test method validation is gauge r&r (repeatability and reproducibility), a. Document the test method and criteria. The requirement for chemical test method validation can be obtained from. Web test the test method and criteria. Test Method Validation Vs Gage R&R.
From www.researchgate.net
(PDF) Conducting a Gage R & R Study An Application Example in Test Method Validation Vs Gage R&R Web one of the critical aspects of test method validation is gauge r&r (repeatability and reproducibility), a. The requirement for chemical test method validation can be obtained from. Document the test method and criteria. Web gage repeatability and reproducibility (gr&r) is defined as the process used to evaluate a gauging instrument’s accuracy by. Some ways of performing a test method. Test Method Validation Vs Gage R&R.
From www.lambdatest.com
Verification vs Validation Know The Differences in Testing Test Method Validation Vs Gage R&R Document the test method and criteria. Some ways of performing a test method validation include (but is not. Confirm that the gage r&r in the study is close to 100 percent. Web to compensate for the lack of agency guidance and still meet regulatory requirements, the medical device industry turned to. Web gage repeatability and reproducibility (gr&r) is defined as. Test Method Validation Vs Gage R&R.
From ppt-online.org
Method Validation and Verification Protocols for Test Methods Test Method Validation Vs Gage R&R Web gage repeatability and reproducibility (gr&r) is defined as the process used to evaluate a gauging instrument’s accuracy by. Web to compensate for the lack of agency guidance and still meet regulatory requirements, the medical device industry turned to. Web test method validation is the documented process of ensuring a test method is suitable for its intended use. The requirement. Test Method Validation Vs Gage R&R.
From www.youtube.com
Gage R&R Study Presentation YouTube Test Method Validation Vs Gage R&R Web test method validation is a broader term. Web one of the critical aspects of test method validation is gauge r&r (repeatability and reproducibility), a. Document the test method and criteria. Web to compensate for the lack of agency guidance and still meet regulatory requirements, the medical device industry turned to. Some ways of performing a test method validation include. Test Method Validation Vs Gage R&R.
From vancouverwool.web.fc2.com
Anova Gage R And R Test Method Validation Vs Gage R&R Web test the test method and criteria (the operational definition) with some test samples (perform a gage r&r study). The requirement for chemical test method validation can be obtained from. Web to compensate for the lack of agency guidance and still meet regulatory requirements, the medical device industry turned to. Web gage repeatability and reproducibility (gr&r) is defined as the. Test Method Validation Vs Gage R&R.
From in.pinterest.com
Validation vs Verification Explained Test Method Validation Vs Gage R&R Web gage repeatability and reproducibility (gr&r) is defined as the process used to evaluate a gauging instrument’s accuracy by. Web test method validation is the documented process of ensuring a test method is suitable for its intended use. The requirement for chemical test method validation can be obtained from. Some ways of performing a test method validation include (but is. Test Method Validation Vs Gage R&R.
From www.researchgate.net
The results of Gage R&R (crossed) Analysis Download Scientific Diagram Test Method Validation Vs Gage R&R The requirement for chemical test method validation can be obtained from. Web test method validation is the documented process of ensuring a test method is suitable for its intended use. Some ways of performing a test method validation include (but is not. Web test method validation is a broader term. Web gage repeatability and reproducibility (gr&r) is defined as the. Test Method Validation Vs Gage R&R.
From www.sifo-medical.com
Test Method Validation of Continuous Destructive Measurements (Gage Test Method Validation Vs Gage R&R Web test method validation is the documented process of ensuring a test method is suitable for its intended use. Document the test method and criteria. Web one of the critical aspects of test method validation is gauge r&r (repeatability and reproducibility), a. Web gage repeatability and reproducibility (gr&r) is defined as the process used to evaluate a gauging instrument’s accuracy. Test Method Validation Vs Gage R&R.
From sixsigmastudyguide.com
Gage Repeatability and Reproducibility (R&R) Test Method Validation Vs Gage R&R The requirement for chemical test method validation can be obtained from. Web gage repeatability and reproducibility (gr&r) is defined as the process used to evaluate a gauging instrument’s accuracy by. Web test the test method and criteria (the operational definition) with some test samples (perform a gage r&r study). Web one of the critical aspects of test method validation is. Test Method Validation Vs Gage R&R.
From www.fierceelectronics.com
Verification and Validation Upping your Quality Management Game Test Method Validation Vs Gage R&R Web test the test method and criteria (the operational definition) with some test samples (perform a gage r&r study). Document the test method and criteria. Web test method validation is a broader term. Web test method validation is the documented process of ensuring a test method is suitable for its intended use. Some ways of performing a test method validation. Test Method Validation Vs Gage R&R.
From www.slideserve.com
PPT Analytical Methods What, When and How to Validate PowerPoint Test Method Validation Vs Gage R&R Confirm that the gage r&r in the study is close to 100 percent. Some ways of performing a test method validation include (but is not. Web test method validation is a broader term. Web one of the critical aspects of test method validation is gauge r&r (repeatability and reproducibility), a. Web to compensate for the lack of agency guidance and. Test Method Validation Vs Gage R&R.
From fivevalidation.com
Verification & Validation V&V FIVE Validation Test Method Validation Vs Gage R&R Web test method validation is the documented process of ensuring a test method is suitable for its intended use. Web gage repeatability and reproducibility (gr&r) is defined as the process used to evaluate a gauging instrument’s accuracy by. Web one of the critical aspects of test method validation is gauge r&r (repeatability and reproducibility), a. Document the test method and. Test Method Validation Vs Gage R&R.
From galaxyinferno.com
What is validation data used for? Machine Learning Basics Galaxy Test Method Validation Vs Gage R&R Some ways of performing a test method validation include (but is not. Confirm that the gage r&r in the study is close to 100 percent. Web one of the critical aspects of test method validation is gauge r&r (repeatability and reproducibility), a. Web test the test method and criteria (the operational definition) with some test samples (perform a gage r&r. Test Method Validation Vs Gage R&R.
From validationcenter.com
What is Computer System Validation and How Do You Do It? Test Method Validation Vs Gage R&R Web one of the critical aspects of test method validation is gauge r&r (repeatability and reproducibility), a. Some ways of performing a test method validation include (but is not. Web test method validation is the documented process of ensuring a test method is suitable for its intended use. Web test method validation is a broader term. Web to compensate for. Test Method Validation Vs Gage R&R.
From sanahylos12.blogspot.com
R&R Meaning / Gage R R Ppt Download Video shows what r&r means Test Method Validation Vs Gage R&R The requirement for chemical test method validation can be obtained from. Confirm that the gage r&r in the study is close to 100 percent. Web to compensate for the lack of agency guidance and still meet regulatory requirements, the medical device industry turned to. Web test method validation is the documented process of ensuring a test method is suitable for. Test Method Validation Vs Gage R&R.